Dr. Robert Malone's Comment Tonight Caught my Attention
This is about placing the host at increased risk of ADE and COVID-19 Severity
Although the discussion tonight was around the labelling of other respiratory illnesses/deaths as COVID-19, there was a point made tonight by Dr. Robert Malone that caught my attention.
https://childrenshealthdefense.org/defender/rfk-jr-podcast-flu-data/?utm_source=salsa&eType=EmailBlastContent&eId=e05509d1-c79c-4bbf-a561-6fe664895c6e
That is that during natural infection with SARS-CoV-2 the spike specific antibodies arise much sooner than expected for a primary exposure (10-14 days rather than 21-28 days). This is important because innate immunity needs a good 10 days to clear out SARS-CoV-2. If the adaptive spike specific antibodies (neutralizing and enhancing) are produced prior to clearance of SARS-CoV-2 by innate immunity, then via antibody dependent enhancement (ADE), the all important WDR74 positive foamy macrophages get preferentially infected and the host looses the most critical part of the immune system that defends against pandemic viruses [Ren X et al, Cell 2021]: trained innate immunity involving HERV-K102 particle production. Instead progression to severe disease and death would be more likely.
In my submitted paper there is a section that talks about this issue. Here it is.
The BCG Vaccine Clinical Trials
BCG vaccines protect non-specifically against infectious diseases caused by respiratory viruses [1,2]. In a small randomized clinical trial (RCT) involving participants 65 years of age and older (n=198), those that received the BCG vaccine showed a decreased risk of any infection with any pathogen but where this protection was more pronounced for respiratory infections [3]. In vitro, significantly elevated levels of TNFa, IL-6, IL-10 and IFNg were shown in PBMCs in response to various antigens when the vaccinated were compared with the unvaccinated. Additionally, the levels of H3K27ac were elevated in the promoters of IL-6 and TNFa showing trained immunity. However, in an expanded follow-up study involving respiratory tract infections (n=2014), no benefit was observed as the BCG vaccine trial was conflated by 81 % of the participants being vaccinated with COVID-19 vaccines [4]. Interestingly, BCG vaccinated participants who became infected with SARS-CoV-2 showed significantly higher IgG responses to SARS-CoV-2 S protein and the receptor binding domain (RBD) than those who did not receive the BCG vaccine [4]. This enhancement appeared to indicate that BCG induced trained immunity may prime also for adaptive immunity which might advance the onset and levels of spike specific antibodies produced with potentially grave consequences. It remains to be seen if prior recent vaccinations such as the influenza vaccination might contribute to increased risk of COVID-19 severity.
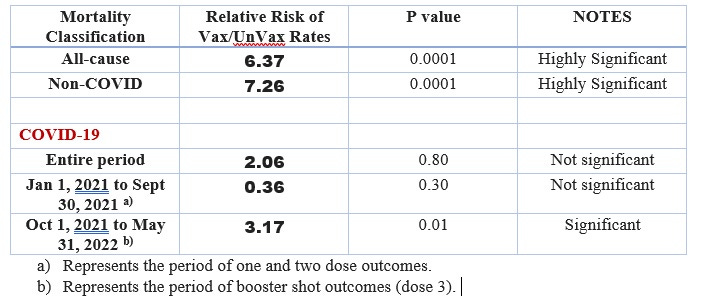
This is EXTREMELY IMPORTANT TO RESOLVE as outcomes in the elderly populations to natural infections could be jeopardized by stimulating an earlier IgG response (ie., earlier responders) which then mediates ADE leading to more severe disease. In other words this is dangerous if the host has not had sufficient time to clear the virus by innate immunity. Is this phenomenon a contributing factor to why in January 2021 with the first dose (in which SARS-CoV-2 was commonly transmitted at the first dose vaccination clinics) these vaccinated elders had higher mortality rates over the unvaccinated (See Image 1). Statistics for the ratios are provided in Image 2.
IMAGE 1. Results for January 2021 in the UK showing increased COVID-19 and non-COVID-19 mortality rates in the vaccinated over the unvaccinated (primarily older adults for January 2021). The increased COVID-19 mortality is thought to be due in part to increased SARS-COV-2 transmission at the large indoor COVID-19 vaccine clinics. However as argued here, some of this increased risk for mortality may have resulted from being recently vaccinated with influenza or other vaccines which may have caused “early responders”. Early responders were also shown for SARS (SARS-CoV-1) where the antibodies to spike were detected by two weeks (instead of after 5 weeks) and had a higher death rate and shorter survival times [Ho MS, EID, 2005].
Note that with time the relative risk increases across the board implying damage done by vaccination is cumulative and/or making pandemic mortality worse due to vaccine selection of variants and greater risk of ADE.
Image 2. Statistics show that the COVID-19 vaccines:
failed the all-cause mortality test meaning the rate of all-cause deaths in the vaccinated was significantly elevated (6.37-fold and highly significant) over the unvaccinated and the product should not have been marketed due to significantly higher risk of death over any benefit;
failed to show effectiveness of significantly reducing non-COVID-19 deaths for the entire period January 1, 2021 to May 31, 2022 (7.26-fold and highly significant), vaccines associated with non-COVID-19 mortality;
failed to show effectiveness of significantly reducing COVID-19 deaths for the entire period January 1, 2021 to May 31, 2022 (2.06-fold but not significant);
failed to show effectiveness of significantly reducing COVID-19 deaths for January 1, 2021 to September 30, 2021 related to one or two doses of vaccine (0.36-fold but not significant);
failed to show effectiveness of significantly reducing COVID-19 deaths for period covering October 1, 2021 to May 31, 2022 related to third doses of vaccine in October 2021 (3.17-fold and significant at p<0.05).
Other interesting insight from the BCG vaccines
COVID-19 infections may be less prevalent in countries with universal BCG vaccination (possibly also higher endemic levels of M. tuberculosis) and so there are at least 56 randomized clinical trials (RCTs) underway to assess the efficacy of this innate immunity vaccine to protect against SARS-CoV-2 infection [1,2]. In a cohort study of booster BCG vaccination in the United Arab Emirates of hospital workers in early March of 2020, by the end of June 2020, none of the 71 participants who received the booster (0%) became infected with SARS-CoV-2 whereas there were 18 infections in the not boosted controls (8.6 %) [5]. However, one of the first reports from a double blind, phase 3 RCT (n=1000) conducted from May 2020 to October 2021, claimed the BCG vaccine did not protect healthcare workers against SARS-COV-2 infection nor hospitalization [6]. Remarkably, 70 % of the placebo group and 66 % of the BCG vaccine group received the COVID-19 vaccine (dosing and time of dosing was not revealed for either arm), and another 5% and 4% (respectively) also received influenza vaccination during the study. The correct interpretation of the study is that this was a mistrial where the benefits and risks of BCG could not be evaluated given that greater than 70% of all participants received other vaccines especially COVID-19 vaccines, during the study. Unfortunately, it is expected that many more BCG vaccine RCTs will be similarly disqualified and misclassified as ineffective when in fact the RCT was compromised by concomitant vaccination. One might question if this was intentional as none of the COVID-19 vaccines represented the standard of care as they only had emergency use authorization and thus, should have been delayed until the end of the BCG trial. This is potentially another sneaky way that Big Pharma creates and then spreads misinformation, and/or ensures competing “off-label” biologics are sabotaged.
1. Wang J, Zhang Q, Wang H, Gong W. The potential roles of BCG vaccine in the prevention or treatment of COVID-19. Front Biosci (Landmark Ed). 2022 May 13;27(5):157. doi: 10.31083/j.fbl2705157.
2. Gonzalez-Perez M, Sanchez-Tarjuelo R, Shor B, Nistal-Villan E, Ochando J. The BCG vaccine for COVID-19: first verdict and future directions. Front Immunol. 2021 Mar 8;12:632478. doi: 10.3389/fimmu.2021.632478.
3. Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020 Oct 15;183(2):315-323.e9. doi: 10.1016/j.cell.2020.08.051.
4. Moorlag SJCFM, Taks E, Ten Doesschate T, van der Vaart TW, Janssen AB, Müller L, et al. Efficacy of Bacillus Calmette-Guérin vaccination against respiratory tract infections in the elderly during the Covid-19 pandemic. Clin Infect Dis. 2022 Mar 5:ciac182. doi: 10.1093/cid/ciac182.
5. Amirlak L, Haddad R, Hardy JD, Khaled NS, Chung MH, Amirlak B. Effectiveness of booster BCG vaccination in preventing Covid-19 infection. Hum Vaccin Immunother. 2021 Nov 2;17(11):3913-3915. doi: 10.1080/21645515.2021.1956228.
6. Upton CM, van Wijk RC, Mockeliunas L, Simonsson USH, McHarry K, van den Hoogen G, et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine. 2022 Jun;48:101414. doi: 10.1016/j.eclinm.2022.101414.






This is a great article, thank you for posting it!
In Shrock E, Fujimura E, Kula T, Timms RT, Lee IH, Leng Y, et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020 Nov 27;370(6520):eabd4250. doi: 10.1126/science.abd4250. Cross reactivity to other CoV and reactivation of CMV and/or HSV-1 seemed to place the host at higher risk of hospitalization consistent with the 'early response' hypothesis (early spike IgG causing ADE and progression due to cross-priming).