PART II: HOW SARS-COV-2 TARGETS HERV-K102 IN SEBOCYTES AND FOAMY MACROPHAGES
Inhibiting HERV-K102 Particle Production and Release Resulting in Increased SARS-CoV-2 Replication and Pathogenesis
Updated March 3 2022 ori January 28, 2022
Note: it is difficult to say what type of epithelial cell was found in the saliva as staining for spike protein or RNA was not performed on sebaceous glands and sebocytes express the pCK marker used to identify the epithelial cell.
As outlined in Figure 9, SARS-CoV-2 might utilize several mechanisms to counteract the HERV-K102 protector system against pandemic viruses (see description of HERV-K102 Innate Protector System in Part I January 26, 2022 ). HERV-K102 particle production and release seems constitutive in sebocytes (specialized foamy macrophages in sebaceous glands in the mucosa). HERV-K102 is also induced in foamy macrophages such as in the lungs in patients with moderate or severe COVID-19. SARS-CoV-2 appears to target both sebocytes and foamy macrophages as discussed in this Part II.
Figure 9. Overview of Six Mechanisms by Which SARS-CoV-2 May Target and Inhibit the HERV-K102 Innate Immunity Protector System
Figure 10 documents the evidence that SARS-CoV-2 targets foamy sebocytes and/or macrophages through basigin (BSE/CD147).
Figure 10. SARS-CoV-2 May Target Sebocytes and Foamy Macrophages through a Universal Receptor Basigin (BSG/CD147) and may Disrupt Foam Cell Formation
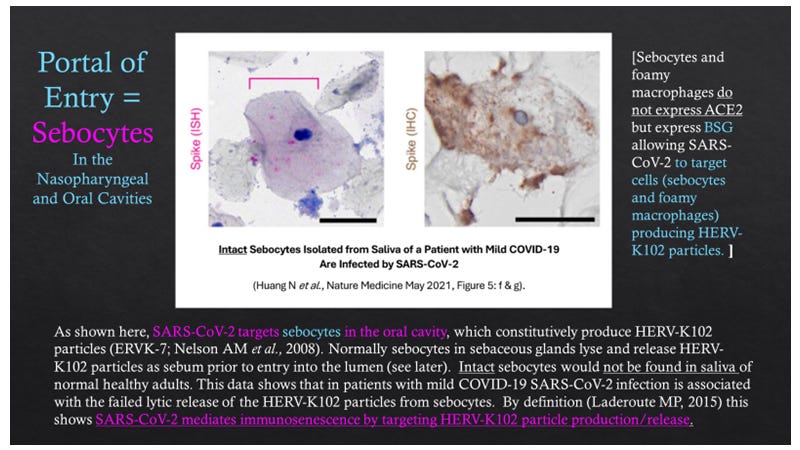
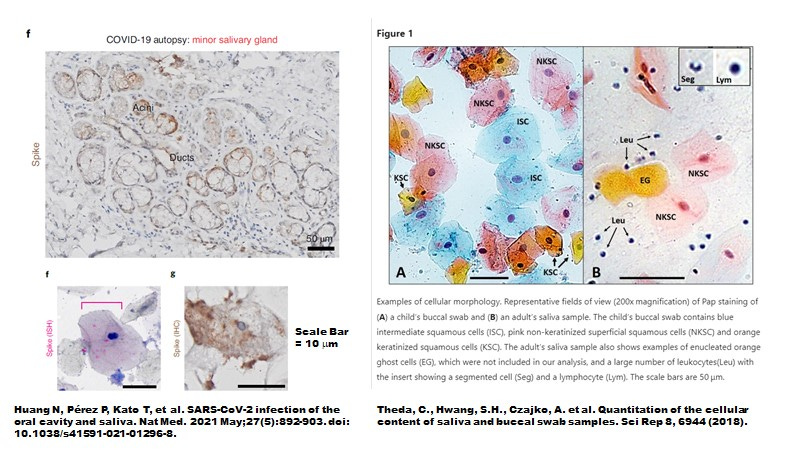
Indeed, while intact sebocytes do not seem to be shed in normal healthy saliva, in patients with mild COVID-19, intact sebocytes from saliva are in fact infected with SARS-CoV-2 as shown by in situ hybridization (ISH) for spike RNA and by immunohistology for spike protein (Figure 11 from Huang N et al., Nature Medicine, 2021).
Figure 11. In Patients with Mild COVID-19, Intact Epithelial Cells Resembling Sebocytes Are Infected by SARS-CoV-2
The evidence supporting the notion that SARS-CoV-2 inhibits the interferon gamma (IFN-G) and/or IRF-1 response which is needed for HERV-K102 particle production is provided in Figure 12.
Figure 12. Evidence Supporting That SARS-CoV-2 Inhibits IFNG/IRF1 Induction Of HERV-K102 Transcripts and/or Foam Cell Formation
DDX6 needed for the transport of HERV-K102 particles from the nucleus to the cytoplasm is inhibited by SARS-CoV-2 (Figure 13).
Figure 13. SARS-CoV-2 Inhibits DDX6.
SARS-CoV-2 promotes the malignant/virulent phenotype blocking HERV-K102 particle production (Figure 14).
Figure 14. SARS-CoV-2 Promotes the Malignant/Virulent Phenotype
While SARS-CoV-2 may upregulate AFP mRNA and protein expression, it may also enhance TGF-B1 suspected of specifically binding and activating AFP. As it is the activity of AFP which causes immunosenescence these cofactors may further exacerbate immunosenescence blocking the release of HERV-K102 particles (Figure 15).
Figure 15. SARS-CoV-2 Promotes Apoptosis Resistance Due to AFP Activity
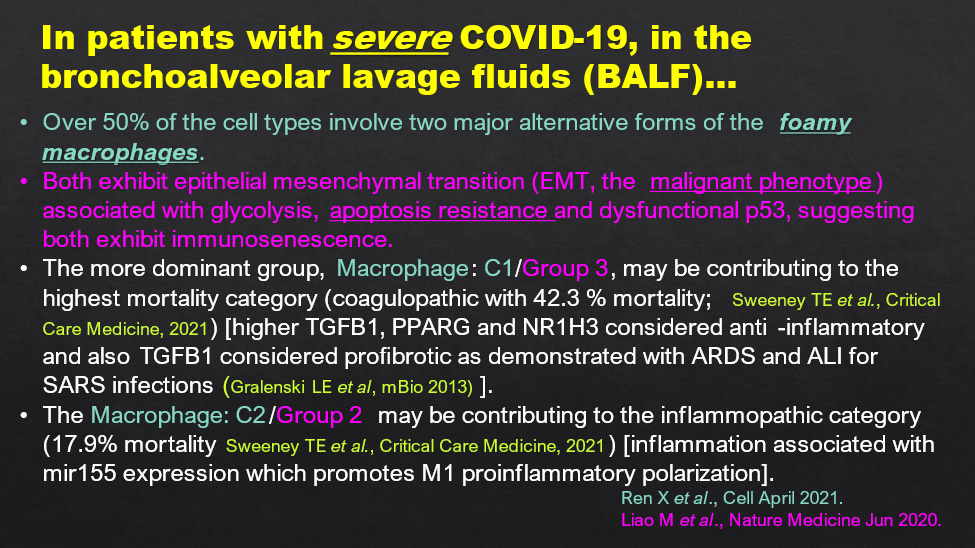
Consistent with the notion of active AFP mediating apoptosis resistance, Liao et al (2020) have described apoptosis resistance in the two major (foamy) macrophage lineages which dominate in the BALF of severe COVID-19 patients (Figure 16). As well Ren et al., 2021 have shown SARS-CoV-2 RNA inside these macrophages. Apoptosis resistance would block the lytic release of the protector HERV-K102 particles from the foamy macrophages and thus be associated with immunosenescence. This would led to progression of COVID-19 severity but may also contribute to hypertension and risk of cardiovascular disease (see Laderoute M, Discovery Medicine, 2020) as well as other diseases including cancer. This is why it is important to examine all cause mortality in assessment of COVID-19 disease and for SARS-CoV-2 vaccines.
Figure 16. Two Major Types of Macrophages in sBALF Both Showing Apoptosis Resistance
While sebocytes morphologically appear to be identical to foamy macrophages, express the key markers of foamy macrophages including CD14 and CD16 along with the two other Fc receptors and HERV-K102 (ERVK-7) RNA, and undergo apoptosis on day 7 to lytically release their contents; Toroscik D et al., 2018 have demonstrated the immunocompetance of sebocytes (Figure 17). This established that sebocytes are indeed functional foamy macrophages. This strongly supports the notion that sebocytes may be specialized foamy macrophages in sebaceous glands of mucosal tissues constitutively producing and releasing the ‘waxy’ HERV-K102 particles as sebum as a first line of defense.
It should be appreciated that many drugs have been developed to counteract ‘acne’. The main hormones causing acne include androgens, insulin and insulin-like growth factor-1 (IGF-1) and involve various pathways including NF-kb. However, the IGF-1 induced PI3K/Akt/mTOR pathway is the most important for acne pathogenesis (Cong TX et al., Arch Dermatol Res 2019). Commonly used anti-acne agents include retinoids, hormonal agents (spirolactone, combination oral contraceptives and flutamide) and newer agents include PPARG modifiers, melanocortin receptor antagonists, metformin, epigallocatechin and others. All anti-acne drugs attenuate the Akt/mTORC1 signalling and restore p53 signal transduction. As mentioned above, p53 downmodulates AFP involved in the malignant phenotype, virulence and immunosenescence.
Figure 17. Sebocytes Function as Macrophages
Indeed as tested in nasopharyngeal swabs there is evidence for the induction of the HERV-K102 pathway in those samples with low SARS-CoV-2 viral loads which may be thwarted with higher viral loads (Figure 18).
Figure 18. Evidence for the HERV-K102 Induction Pathway in NPS of Mild COVID-19
As shown in Figure 19, there may be anecdotal evidence for the effects of one versus two doses of the Pfizer vaccine in triggering the HERV-K102 innate immunity system in sebocytes as interpreted for blistering of fordyce spots. In future blogs, we will discuss how HERV-K102 particle production fits with the notion of trained innate immunity (a heterologous response protective against a wide variety of intracellular pathogens including viruses that shows memory).
Figure 19. Fordyce Spots and Anecdotal Evidence of Enhancement of HERV-K102 Lytic Release from Sebocytes Associated with COVID-19 Vaccination
CONCERNS ABOUT ADE and HOW This May Exacerbate the Loss of the HERV-K102 Protector System Against Pandemic Viruses
It is commonly said that antibody dependent enhancement (ADE) of emerging pathogens may both increase viral replication allowing for increased infection and transmission, as well as pathogenesis. While this seems to be the case for delta, for omicron we see the latter is not necessarily true, as it does not seem to lead to pneumonia/cytokine storm and thus is less lethal. Current data suggests omicron may have been derived from the house mouse and potentially may be the strain of SARS-CoV-2 (highly mutated) which will help end the pandemic. Nevertheless as mentioned earlier, pre-omicron strains of SARS-CoV-2 once inside these sebocytes/foamy macrophages not only shuts down HERV-K102 particle production (allowing for increased SARS-CoV-2 replication) but by interfering with the lytic release of particles (hyperactivating the PI3K/Akt/mTOR pathway and rendering p53 dysfunctional and thus more AFP produced) contributes to immunosenescence which itself initiates or causes the progression of chronic illness, including COVID-19 severity. Thus, generally ADE is expected to be associated with increased pathogenesis in the host, as compared with infection without ADE. This is a new paradigm to explain the ADE phenomena (manuscript in preparation).
ADE is a known issue for emerging RNA viruses as they mutate relatively quickly and thus antibody selected variants tend to appear with time during pandemics. In particular, ADE is a common concern of coronaviruses (Figure 20).
Figure 20. ADE and SARS-CoV-2 Infection
Various lines of evidence have confirmed ADE is an issue during SARS-CoV-2 natural infection (Figures 20, 21) and as an unintended consequence of vaccination especially when only spike protein is used as the immunogen (Figure 21). Some examples of this evidence are provided in Figures 22 and 23.
Figure 21. ADE and Safety Concerns
Figure 22. Infection Enhancement/ADE Associated with Two Doses of COVID-19 with the Delta Variant in England
Perhaps the most unsettling of the use of the spike specific vaccines for mass vaccination (adaptive immunity vaccination inducing antibody to spike protein) against an emerging RNA virus is that there appears to be selection for escape mutants (we will examine this further in a future blog) and so the whole point of mass vaccination to generate herd immunity or sterilizing immunity is quickly lost as shown in Figure 23. Unfortunately, as may be evident for the delta variant as studied in England, this may increase ADE (Figure 22) as well as pathogenesis in the unvaccinated, partially vaccinated and fully vaccinated (Figure 24).
Figure 23. Vaccine Effectiveness Diminishes with 2 Dose>6 Days but not with 2 Dose <7 days Where Instead Sterilizing Immunity is Associated with the Alpha and Delta Variants
Note: Results are consistent with the anecdotal results in Figure 19, suggesting HERV-K102 particle release and/or antibodies to HERV-K102 may provide sterilizing immunity (ie., under 7 days following the second dose) but which is abrogated when ADE from the adaptive antibodies to spike protein become boosted.
Figure 24. The Evolution of SARS-CoV-2 Delta in England Led to Increased Pathogenesis as Shown by the Increases in Case Fatality and Hospitalization Rates with Time and as Stratified by Age and Vaccination Status
Note: an almost universal finding was that one dose immunization provided the best protection against the delta variant and was independent of age. This is consistent with the notion that fighting pandemics should favor innate immunity vaccines instead of adaptive immunity ones. One dose of Pfizer mRNA vaccines does not produce significant amounts of neutralizing antibodies and very little other S -specific antibodies (Walsh EE et al., NEJM, 2020) and thus, does not provide selective pressure for the emergence of new variants and thus reduces the risk of ADE.
Summary and Conclusions:
We have learned here how SARS-CoV-2 targets sebocytes and foamy macrophages preventing the production and/or release of the HERV-K102 particles and at the same time inducing immunosenescence associated with viral virulence/pathogenesis. The loss of the protector HERV-K102 system may relate to poor viral replication control while the induction of immunosenescence may contribute to COVID-19 pathogenesis. We have uncovered some evidence consistent with the notion that innate immunity potentially pertaining to HERV-K102 particles/antibody may confer sterilizing immunity but which is abrogated with boosting of adaptive immunity. In other words it appears that ADE will counteract or overpower innate immune protector mechanisms. However, clearly more research is needed in this regard.
In future posts, we will have a look at innate immunity versus the adaptive immunity vaccines and the safety of spike containing vaccines.

















Naltrexone, an opioid receptor antagonist, used at low dose (1-5 mgs) is part of the FLCCC i-Recover Protocol (January 19, 2022). In murine models involving adipose tissue macrophages, it acts to block LPS induction of proinflammatory mediators such as IL-6 indicating it may be a TLR4 antagonist (Choubey A et al., J Biomolecular Structure and Dynamics, Sept 2020) and potentially may useful to tame inflammation in COVID-19 or long haul patients. Interestingly, in hepatocellular carcinoma cells (HCC), agents able to block the mu-opioid receptor suppresses HCC via CD147-p53-MAPK cascade (Zhang JJ et al., Am J Transl Res May 2021) implying naltrexone may also reverse immunosenescence in sebocytes and foamy macrophages. Finally, azithromycin, a broad spectrum antibiotic against gram positive bacteria used against malaria, may block the CD147- spike interaction (Ulrich H & Pillat MM. Stem Cell Rev Rep June 2020) preventing the CD147 mediated proinflammatory induction in COVID-19 patients by SARS-CoV-2 (Geng J et al., Signal Transduction Target Therapy, 2021). Both agents then, might be useful for inclusion in the early treatment protocol in the 'test and treat' strategy to end the pandemic.