Completed & Submitted a Literature Review Proposing HERV-K102 as the Elusive Correlate of Protection Against SARS-CoV-2 Infection
May 20, 2022 Version 2.
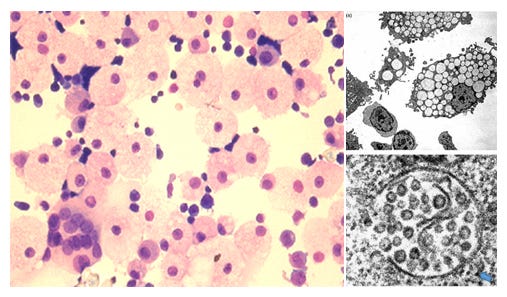
Figure 1. The Production of HERV-K102 Particles in Macrophages Generates M1 Lipid Body Negative Inflammatory FOAMY Macrophages That 1) May Promote Recovery from Moderate COVID-19 and 2) Depletion is Associated with Progression to Severity
My absence from writing on substack over the past few months was because I was writing an invited review on ‘new paradigms in vaccinology’ which turned out to be a much larger task than what I had anticipated. So after 204 references, 11 figures, 1 table and 105 pages later, it was submitted late in the evening of May 17, 2022.
As you are well aware, there has been much consternation over the safety of the COVID-19 vaccines with good reason [Seneff S et al, Food and Chemical Toxicity, April 2022]. Equally significant, full vaccination appears to increase the risk of SARS-CoV-2 infection, hardly a strategy to promote herd immunity. Nevertheless the public health authorities blindly and reprehensibly encourage vaccination.
Here is a summary from this review.
Overall, SARS-CoV-2 inhibits the interferon response impeding the early innate immune response. It is important that the host clears SARS-CoV-2 before the B cell response of adaptive immunity becomes activated since the antibodies and neutralizing antibodies (NAbs) promote severe disease and not protection. The main correlate of protection uncovered by the extensive review, is HERV-K102 particle production in foamy macrophages.
While only one reference specifically identified HERV-K102 env, gag, and pol RNA sequences expressed in whole blood of moderate and severe COVID-19 patients [Guo Y et al, Viruses, 2022], they did show an association with the interferon gamma response. The remainder and bulk of the proof was based on the identification of the cells by differentially expressed genes (DEGs) known for: the lipid-body negative foamy macrophages expressing HERV-K102 particles [Stec M et al., J Leukoc Biol 2007] and for sebocytes [Nelson AM et al, J Clin Invest, 2008]. Sebocytes are specialized foamy macrophages in sebaceous glands lining the mucosa which constitutively produce and release the protector particles as a first line of defense. Other DEGS used to establish the cells producing HERV-K102 included DDX6, IRF1, IFNG, NFKB1 known in the induction of HERV-K102 particle production [Manghera M et al., J. Virol 2016], and that the vitamin D receptor (VDR) controls the differentiation of the lipid-body negative M1 pro-inflammatory foamy macrophages to the lipid-body positive M2 anti-inflammatory foamy macrophages [Oh J et al, Circulation, 2009]. As foam cell formation is intrinsically involved in trained immunity and HERV-K102 particle production, DEGS associated with foam cell formation and those involved with trained immunity were also used to identify the macrophage cell types producing HERV-K102 particles.
As well indirect evidence for diminished HERV-K102 replication was found for severe patients and the upregulation of HML-2 envelope with severity [Temerozo JR et al, Microbiome April 2022]). The latter implies there were more SARS-CoV-2 infected cells associated with severity.
The inability of the host to rapidly clear SARS-CoV-2 relates to pre-existing immunosenescence [Laderoute M, Discovery Medicine, 2015, 2020] and/or vitamin D deficiency. Indeed it seems in those with pre-existing immunosenescence such as hypertension, diabetes, chronic obstructive pulmonary disease, Alzheimers disease, cancer, rheumatoid arthritis to name a few, the blocking of HERV-K102 particle release, allows or signals the B cells of adaptive immunity to generate antibodies and NAbs earlier. Indeed, our World in Data [https://ourworldindata.org/coronavirus] considers that the median time of death occurs at 10 days after symptom onset (i.e., without a suitable intervention).
The antibody dependent enhancement (ADE) of disease (in the lungs) is unlike any we have seen before and which oddly, does not appear to involve selection of SARS-CoV-2 immune escape variants. Neutralizing antibodies (NAbs) to spike protein block the interaction of spike with ACE2 (the primary receptor), favoring interactions of the RBD with the secondary receptor BSG (basigin also known as CD147) which is expressed on the myeloid cells (monocytes (blood), macrophages (lungs) and sebocytes in the nasopharyngeal swabs (NPS)). The interaction of spike with BSG appears to be enhanced by the upregulation of FCGR2A. Only the foamy macrophages producing the protector HERV-K102 articles (and monocyte CD14+ CD16+ progenitors) appear to exhibit high levels of BSG and FCGR2A, meaning with severity, these cells are preferentially targeted by SARS-CoV-2. Moreover these cells in vivo are productively infected by SARS-CoV-2 showing it was not due to non-specific phagocytosis and ingestion. Once the foamy macrophages are infected, SARS-CoV-2 employs a stunning multitude of mechanisms which block HERV-K102 particle production and release. SARS-CoV-2 even blocks the recruitment of monocytes to the lungs needed for the replacement of foamy macrophages programmed to undergo cell lysis by interfering with the release of CCL2.
The entry of SARS-COV-2 through BSG and/or as may be assisted by FCGR2A in macrophages appears to cause cytokine storm, acute respiratory distress (ARD), and eventually diffuse alveolar damage (DAD) increasing the risk of death. Geng et al [Geng J et al, Signal Transduct Target Ther September 2021] have shown that only human BSG transgenic mice show severe pneumonia and not the non-transgenic littermates, while a monoclonal (blocking) antibody to BSG inhibits infection and cytokine storms of SARS-CoV-2 wild type, alpha, beta, delta, and gamma variants in the human BSG transgenic mice. (Omicron was not tested as it had not yet appeared at the time these experiments were done). Note that omicron does not appear to have high affinity for human BSG thus, explaining the lower risk of severe or critical disease with this variant that seems to have evolved in the house mouse [Wei C et al, J Genet Genomics December 2021]. It should be noted that an exploratory Phase 2 trial of the blocking BSG monoclonal antibody ‘meplazumab’ showed promise in human subjects with COVID-19 [Bian H et al, Signal Transduct Target Ther, May 2021].
Accordingly agents or interventions which may prevent or reverse immunosenescence would be the best strategy to reduce infection, hospitalization and/or COVID-19 severity. Agents which would abolish immunosenescence are alpha-fetoprotein (AFP) antagonists like zinc, flavonoids and especially ivermectin [Laderoute MP, Open Heart, April 29, 2021 see https://openheart.bmj.com/content/8/1/e001655.responses#ivermectin-may-prevent-and-reverse-immunosenescence-by-antagonizing-alpha-fetoprotein-and-downmodulating-pi3k-akt-mtor-hyperactivity]. Ivermectin has an amazing and scientifically validated effectiveness in the prophylaxis and treatment of COVID-19 [Kory P et al, J Clin Med Res, February 2022] . A number of groups [McCullough PA et al, The American Journal of Medicine, January 2021; Kory P et al, Am J Therapeutics, April 2021], have developed and successfully employed protocols involving these AFP antagonists in the treatment of patients with COVID-19.
Since these recommended first-line protocols do not employ antivirals or monoclonal antibodies to spike protein, they do not select for resistance/variants. Rather they operate to improve host innate immune responses namely the trained immunity HERV-K102 protection system in foamy macrophages which correlates with recovery from moderate COVID-19 and becomes depleted with progression to severity. Thus, these protocols can be used for any pandemic RNA virus, and is a key strategy for pandemic preparedness.
The importance of vitamin D in COVID-19 pathogenesis is two-fold. First it blocks the excessive inflammation associated with cytokine storm [Tallum A et al, PLoS One, 2016]. Second, it prevents lipid body negative M1 foamy macrophages (which produce the protective HERV-K102 particles) from differentiating into lipid body positive M2 anti-inflammatory foamy macrophages (which do not produce HERV-K102 particles) [Oh J et al, Circulation , 2009]. Interestingly, the conversion of M1 to M2 foamy macrophages is favored in diabetics helping to explain their increased risk.
Remarkably, the foamy macrophages that produce the HERV-K102 particles, i.e., the Macro_c5-WDR74 subset and the cells which differentiate into these foamy macrophages, the Mono_c4 – CD14-CD16 subset, were the only cells in the lungs expressing VDR [Ren X et al, Cell, April 2021]. As well upon infection by SARS-CoV-2 the most upregulated gene was PPARA which again was uniquely expressed by the VDR expressing cells, and which also favors the differentiation of the lipid body negative M1 foamy macrophages (which produce the protective HERV-K102 particles) into lipid body positive M2 anti-inflammatory foamy macrophages (which do not produce HERV-K102 particles).
As HERV-K102 particles induce protector innate T cells, it is notable that also the depletion of innate T cells was also associated with progression to severity [Ren X et al, Cell, April 2021].
Other important highlights from the review are as follows.
The prolific number of mechanisms identified by which SARS-CoV-2 abolishes the HERV-K102 protector system, not to mention the precise targeting of the VDR and PPARA expressing M1 foamy macrophages by SARS-CoV-2, strongly implies significant selection of the Wuhan original strain by passage through BLT humanized mice (BLT= xenografts of human bone marrow, liver, thymus) or a newer version additionally involving xenografts of human lung (BLT-L) which interestingly was published at the time of onset of the pandemic [Wahl A, De C, Abad Fernandez M, Lenarcic EM, Xu Y, Cockrell AS, et al. Precision mouse models with expanded tropism for human pathogens. Nat Biotechnol. 2019 Oct;37(10):1163-1173. doi: 10.1038/s41587-019-0225-9.] This supports the lab-leak theory over recent zoonotic infection. The finding that SARS-CoV-2 devotes much energy to antagonizing the HERV-K102 protection system corroborates the latter as a main correlate of protection.
The development of antibodies and NAbs to spike protein was universally found to correlate with severity and NOT protection. Unexpectantly there were 2 papers that claimed survival from COVID-19 was due to the spike specific NAbs (even though in one of the papers their data proved the NAbs correlated with severity). Instead, factoring in that the innate antibodies to HERV-K102 envelope could be responsible for early neutralization (or as it turns out in the second paper also the HERV-K102 particles in plasma due to how the neutralization experiments were conducted ), in fact the protection correlated with early innate immunity responses and not the spike specific NAbs as claimed [Dispinseri S et al., Nature Communications May 2021; Garcia-Beltran WF et al., Cell January 2021]. A third exception paper was found to be technically flawed and thus uninterpretable [Lucas C et al, Nature Medicine, July 2021]. The publication of these papers in high caliber journals is both baffling and reprehensible.
As mentioned a novel mechanism of ADE in vivo in the lungs was described by Ren et al [Ren X et al, Cell, April 2021]. First only in severe cases but not in moderate cases, were the macrophages (and other BSG positive cells of the immune system) infected. Infection and progression to severity was associated with the enrichment of the B_05-MZB1-XBP1 plasma cells in PBMCs that were found to produce NAb-like antibodies. At this point, infection changed from ACE2 mediated infection (epithelial type cells) to BSG mediated infection of largely myeloid cells implying the inaccessibility of SARS-CoV-2 to the primary receptor allowed for preferential interaction with the secondary receptor, BSG. It remains unclear what role FCGR2A played in the latter except as an enhancing or accessory receptor. Thus, surprisingly, there was likely no selection for variants in vivo associated with severity. Accordingly, this helped to explain the highly disparate findings with respect to the infection of SARS-CoV-2 in monocytes/macrophages in vitro and the involvement of ADE or the lack thereof. It is critical to understand that none of the myeloid cells in vivo that were infected by SARS-CoV-2 expressed ACE2 which was a universal finding. The mapping of the spike RBD amino acid interactions with amino acids of BSG [Helal MA et al, J Biomol Struct Dyn February 2022] implied that contrary to some observations, SARS-CoV-2 interacts strongly with human BSG [Wang K et al, Signal Transduct Target Ther December 2020]. Indeed, severe disease in mice was not observed unless human BSG transgenic mice were utilized [Geng J et al, Signal Transduct Target Ther, September 2021]. This matches with the evolution of a non-severe variant omicron in mice which appears to have lower virulence in humans.
Interestingly, point 3 implies that selection of variants really doesn’t happen in vivo in the lungs associated with severity (unless monoclonal antibodies are used but whether this occurs in the lungs or in the nasopharyngeal compartment will be important to resolve). Moreover, as strange as it sounds, Ziegler et al [Ziegler CGK et al, Cell, September 2021] indicated that the interferon responsive macrophages (which also did not express ACE2) that were infected in vivo by SARS-COV-2 in the NPS (which phenotypically resembled TLR2/TLR4 stimulated sebocytes) completely lacked BSG, FCGR3A (CD16) and the trained immunity enhancers SpI1 and CEBPB [Arts RJW et al, Front Immunology, February 2018] but expressed high levels of FCGR2A. Thus, only in the NPS could there be selection of SARS-CoV-2 variants by ADE of infection involving FCGR2A because apparently they lacked BSG (no secondary receptor to use). It remains to be determined if the downregulation of BSG in the NPS related to peridontitis or some other infection or mechanism which would be important to resolve for pandemic preparedness. Perhaps immunosenescence in the host is associated with reduced BSG in sebocytes? Nevertheless, this finding helps to explain how omicron readily infects fully vaccinated individuals while it presents with less virulence.
This unexpected scenario that selection occurs in the NPS but not in the lungs associated with antibodies and neutralizing antibodies (such as induced by vaccination) was used to explain why it seemed in Canada that the two dose vaccination protocol (but not the first dose only) selected for both the alpha and delta variants. These infected persons were largely spared hospitalization or severe disease due to being vaccinated (presumably the spike specific T cell response protected against hospitalization and severity). But the selected variants spread in the communities.
Even in immunocompromised elderly patients hospitalized for long times with COVID-19, selection of escape mutants did not occur unless treated with monoclonal antibodies [Jensen B, Luebke N, Feldt T, Keitel V, Brandenburger T, Kindgen-Milles D, et al. Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany. Lancet Reg Health Eur. 2021 Sep;8:100164. doi: 10.1016/j.lanepe.2021.100164.] .
The selection of the alpha and delta variants in Canada were associated with a two dose to one dose ratio over 0.5 or with a notable loss of heterologous protection against non-COVID-19 all-cause mortality induced by one dose of the vaccine (determined by examining negative excess all-cause mortality). While one dose induces heterologous protection associated with trained immunity, merely administrating the second dose did not affect this [Xu S et al, MMWR 2021]. Thus, the loss of heterologous protection could only result from SARS-CoV-2 infection of macrophages. Putatively, SARS-CoV-2 infection of macrophages (presumptive sebocytes) in the NPS appeared to reverse heterologous protection which involved the NAbs generated by the second dose of the vaccine.
That the two dose vaccination caused the selection of more virulent variants in Canada [Fisman DN & Tuite AR, CMAJ October 2021] is a serious adverse event of vaccination. Not only does it defeat the purpose of vaccination to increase herd immunity but it causes more morbidity and mortality as the pandemic rages on. It highly unlikely that public health officials wanted more disease caused by vaccination, which had they conducted longer term evaluation of safety (as is required for other vaccines), this travesty could have been avoided.
A study of the timing of infection for those who only received one dose in Canada showed that the bulk of the infections (80%) occurred by day 21. In the test-negative analysis by Chung et al for Ontario the mostly highly populated province in Canada [Chung H et al, BMJ, August 2021], 26 %, 65%, 80% and 97% of the symptomatic SARS-CoV-2 infections occurred at up to 6 days, at 0-13 days, 0 to 21 days and 0 to 34 days respectively after the administration of the first dose of vaccine. This data excluded long term care residents. This strongly implied that transmission occurred at the time of the first dose vaccination which involved many hours spent in lineups indoors with hundreds of people. This would strongly underestimate the vaccine effectiveness (VE) against infection for the one dose. Indeed, the unadjusted % VEs showed no protection against severe disease (hospitalizations and death) until greater than 35 days after the first vaccination dose. The adjusted VE against severe disease (hospitalization and death) was 92% at greater than 34 days for the one dose indicating it was as effective as two doses. Thus, the manufacturers’ clinical trials which administered the second dose around 21 days after the first dose significantly underestimated the vaccine efficacy of the first dose. Two vaccine doses would be better for their business plan, however.
That the one COVID-19 mRNA dose which induces trained immunity (HERV-K102) and provides heterologous protection against all-cause mortality (significantly reduced by about 70%) protects as well as two doses without the selection of variants, provides the ultimate indirect proof that HERV-K102 particle production mediates the prevention and recovery of COVID-19. This just needs to be confirmed by testing for HERV-K102 particles using established ddCt PCR methods for cDNA [Laderoute M et al., AIDS, 2007].
The previous proposal that HERV-K102 particle production is likely also the correlate of protection against another concurrent RNA pandemic virus, HIV-1 [Laderoute M, F1000 Res 2018; see https://f1000research.com/articles/6-868 ], where sterilizing immunity associated with the lack of HIV-1 specific antibodies was shown [Laderoute M et al, Open AIDS J, 2015] strongly endorses the notion that HERV-K102 particle production is the elusive correlate of protection against SARS-CoV-2.
That manufacturers of the COVID-19 vaccines and public health authorities had not established the correlate of protection against SARS-CoV-2 before releasing and let alone mandating 2 dose vaccination protocols and where unexpectantly and inappropriately, vaccine safety surveillance protocols were aborted, speaks loudly to the corruption of the scientific method by monetary interests of Big Pharma as well as public health authorities in particular the NIH, the WHO and including the Public Health Agency of Canada and other such agencies across the world. They all knew better that a safe and effective adaptive immunity, virus specific vaccine against an emerging or pandemic RNA virus was proven time and time again to be impossible.
So in the summary and conclusions I proffered that two dose ADAPTIVE IMMUNITY vaccination against a pandemic RNA virus is not a very good approach to contain a pandemic. If any vaccines were contemplated, only innate immunity vaccines should be considered. Some of these are already in global use such as the live BCG vaccine against tuberculosis.
Instead I suggested that early treatment intervention would be the less costly and the most practical to quickly end a pandemic (and could have prevented the economic and sociological devastation which appears to have eroded the middle class). Rather than SARS-CoV-2 specific ‘anti-virals’ or spike specific monoclonal antibodies which would select for resistance, I reiterated my opinion that the prevention and reversal of immunosenescence would be key to ending the pandemic using the established protocols [Kory P et al, Am J Therapeutics, April 2021; McCullough PA et al, The American Journal of Medicine, January 2021; Kory P et al, J Clin Med Res, February 2022], which have been proven to be highly successful.
As I recommended on March 5, 2020 on LinkedIn before the pandemic was declared, individuals could reduce their risks of COVID-19 severity by reversing immunosenescence with better lifestyle choices, supplementing with zinc and lysine (both considered non-specific anti-virals probably because they allow the release of HERV-K102 particles or support particle production, respectively), flavonoids, vitamins, getting more sunshine and getting fit. Avoiding stress, alcohol and sugar and practicing good sleep hygiene serves to get you there sooner.



Thank you for your work. I am stunned and amazed to read that antibodies, even neutralizing antibodies, promote severe disease. This is certainly not what we have been told by the health bureaucrats. I look forward to more information on this subject.
Yes actually all the medical scientists and vaccinologists and the Brighton Collaboration on Vaccine Safety anticipated (and feared) that antibody dependent enhancement (ADE) of infection into macrophages could spell "big trouble". What was not anticipated was that the ADE mechanism would not provide selection pressure for the generation of variants during natural infection. It turns out the COVID-19 vaccines but not natural infection caused the emergence of the alpha and delta variants (see my post July 1, 2022 which goes through the evidence). Using a vaccine that itself selects for variants is like shooting oneself in the foot. It just prolongs the pandemic, not to mention spike protein is quite toxic.